Science
Injectable Biological Treatment
for DDD
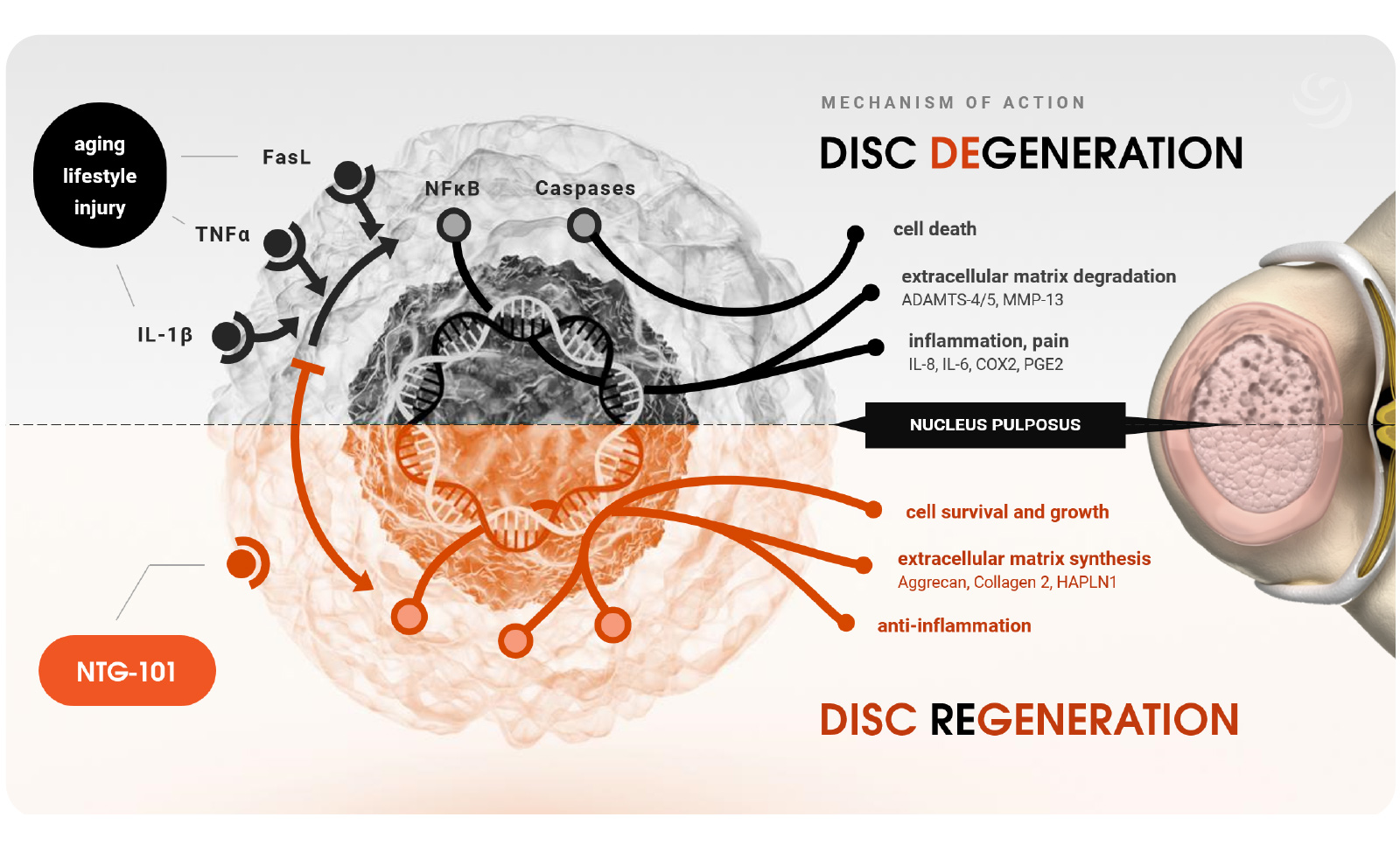
NTG-101 is protein-based biologic designed to mediate the progression of spinal disc degeneration disease (DDD) and induce a restorative effect. Based on more than 20 years of research, this novel therapeutic formulation has the potential to improve the lives of millions of people worldwide suffering from back and neck dysfunction.
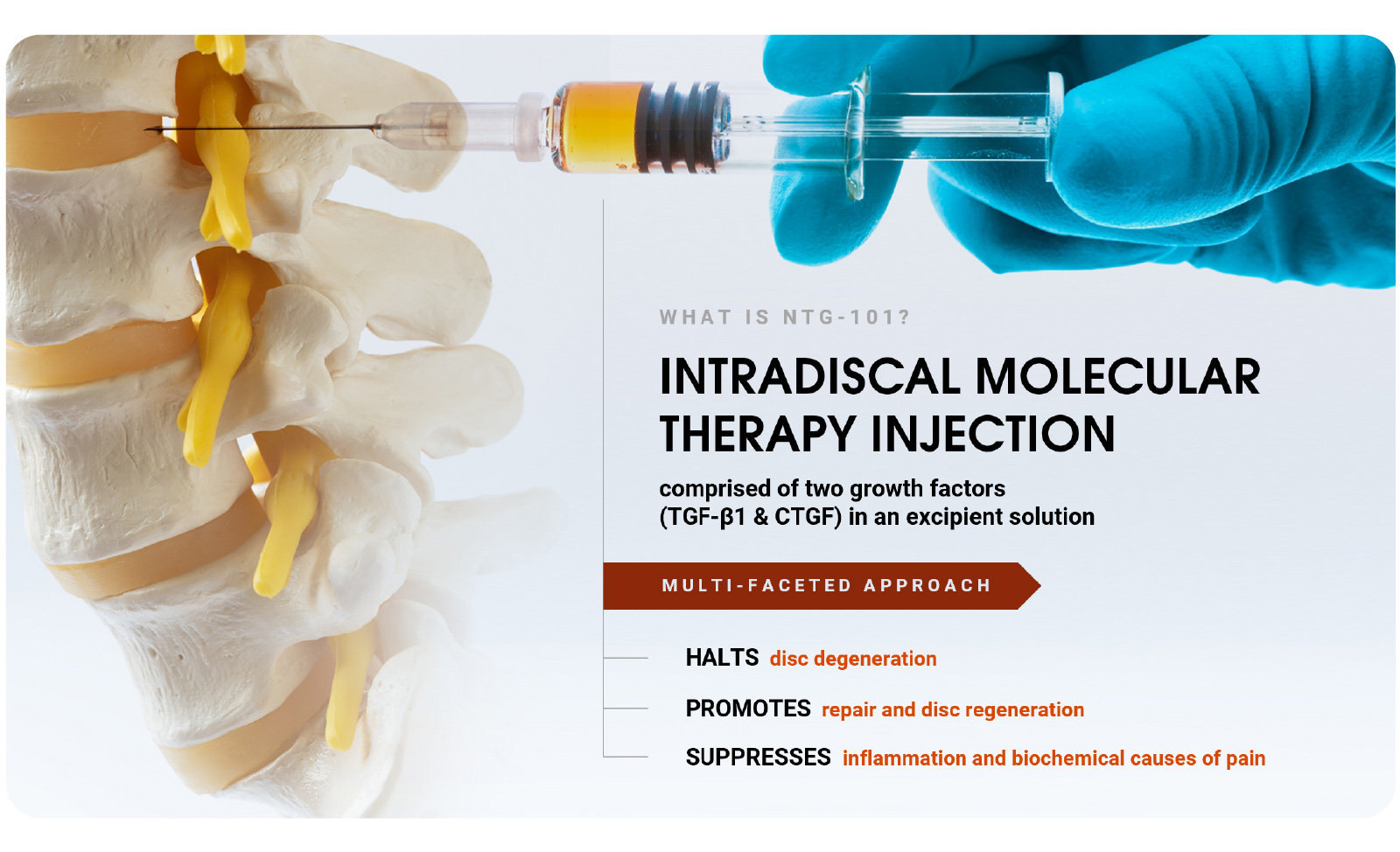
NTG-101 is administered as an intradiscal injection, containing a combination of recombinant human (rh) transforming growth factor beta 1 (TGF-β1) protein plus excipient factors. In preclinical studies, NTG-101 demonstrates robust anti-inflammatory, anti-catabolic and pro-anabolic effects in animal models of DDD.
NTG-101: Protein-Based, Non-Cellular Biologic
- Product Design: A non-cellular biological solution, delivered as an intradiscal injection.
- Development Status: NTG-101 is pre-clinical and is undergoing cGMP manufacturing prior to initiating regulatory submissions and clinical trials.
Development Approach
Notogen’s NTG-101 therapeutic technology was developed through a comprehensive analysis of non-chondrodystrophic animal species which are protected from developing degenerative disc disease and have been the subject of numerous publications from sources worldwide.